“Bringing Effective Treatments to Patients Faster”
 That’s one hope the FDA has for its ISTAND Pilot Program. Toward that goal, the FDA has accepted Integral Molecular’s MPA+IND service as the first Drug Development Tool to be considered under this program. To learn more about the acceptance of our Letter of Intent (LOI), read the FDA’s statement.
That’s one hope the FDA has for its ISTAND Pilot Program. Toward that goal, the FDA has accepted Integral Molecular’s MPA+IND service as the first Drug Development Tool to be considered under this program. To learn more about the acceptance of our Letter of Intent (LOI), read the FDA’s statement.
“We share the FDA’s interest in developing in vitro technologies to better assess the safety of antibody drugs at an earlier and less costly stage of development.”
—Benjamin Doranz, CEO of Integral Molecular
Streamlining Regulatory Review
Tools that qualify under ISTAND will be qualified for regulatory applications without the FDA needing to reconsider and confirm their suitability. You can learn more on the FDA’s ISTAND Pilot Program webpage.
By identifying off-target interactions early in the development process, our Membrane Proteome Array (MPA) platform is already designed to reduce the cost and time it takes to bring new biotherapeutics to patients. And it provides the type of safety profiling data the FDA requires in regulatory submissions for biotherapeutics. Once it qualifies under ISTAND, this platform could streamline your regulatory submission process even further.
Next Steps for ISTAND Qualification
The FDA’s acceptance of our LOI was the first of three steps in the ISTAND qualification process. We are working with the FDA on the next step: a Qualification Plan.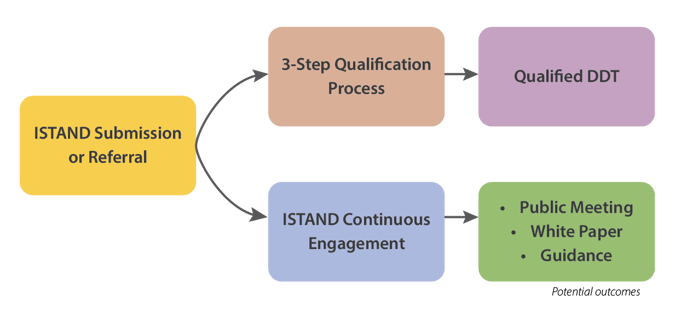
Possible pathways to outcomes under the ISTAND program
(Based on image from FDA)
Stay up to date
If you’d like to receive email updates on our ISTAND qualification process, please enter your information below.