The Need: Antibody Off-Target Screening
Once medullary thyroid cancer becomes metastatic, treatment options are limited and prognosis is poor. A promising newer possibility is to use CAR-T immunotherapy to target medullary thyroid cells. Researchers at the University of Pennsylvania Perelman School of Medicine identified GFR𝛼4 as a protein marker specific to these cells—and to the metastases derived from them. They developed monoclonal antibodies against GFR𝛼4 and identified two potential antibodies to develop as therapeutic candidates.
To choose the best antibody to develop, and ensure the safest immunotherapy possible, the team needed a way to screen them for off-target binding.
The Solution: MPA for Cross-reactivity
The team chose the MPA platform to screen the antibodies for cross-reactivity. The MPA includes thousands of unique human membrane proteins, expressed on unfixed cells. Screening revealed that both antibodies bound strongly to GFR𝛼4. However, one (P4-6) cross-reacted with another protein and was thus eliminated. The other (P4-10) showed no significant off-target binding and was further developed for CAR-T.
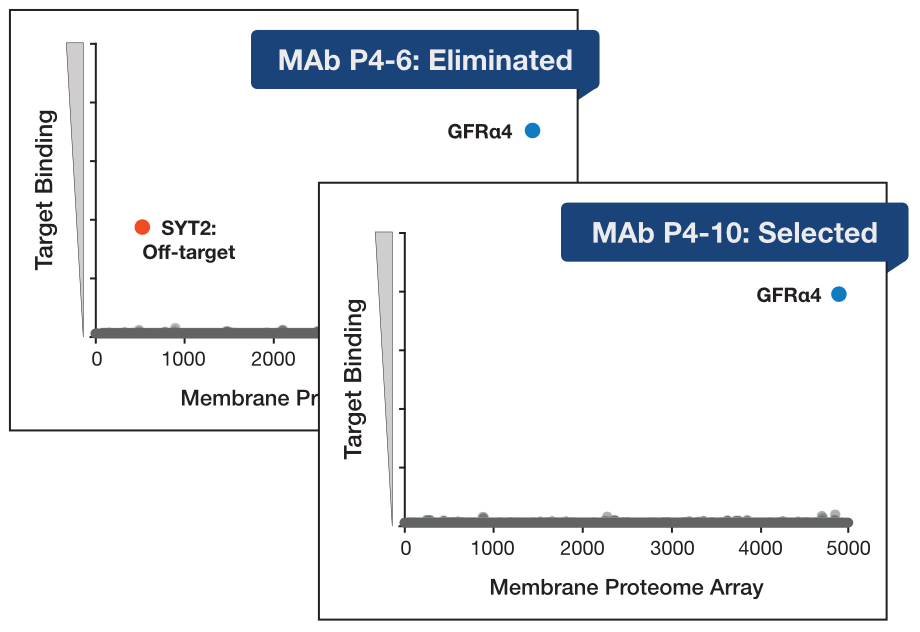
data based on Bhoj et al, 2021 (Fig. S4)
The Impact: Confident Lead Selection
The MPA results helped the team confidently move forward with an antibody that demonstrated no cross-reactivity. The team used the antibody as the basis for a CAR-T immunotherapy, which performed well in further testing.
The new immunotherapy holds promise as an effective treatment option for people with medullary thyroid cancer, who currently have no curative options.
Data featured in Molecular Therapy: Oncolytics, Bhoj et al, 2021
“Integral Molecular’s MPA gave us the data we needed for lead selection. It quickly told us, early in preclinical development, that our molecule had no off-target binding to other human membrane proteins. It also provided important safety data for our IND filing with the FDA, allowing us to move into clinical studies.”
-Don L. Siegel, MD, PhD, Professor of Pathology & Laboratory Medicine, University of Pennsylvania Perelman School of Medicine
Clinical Development
In August 2021, the CAR-T therapy moved into a phase 1 open-label study. It is being evaluated for safety and feasibility in patients with incurable, metastatic medullary thyroid cancer. NCT04877613
Contact Us
Specificity Testing of Antibody and CAR-T Cell Therapies for IND Using the Membrane Proteome Array
To hear Don Siegel speak about this case study, including the personal story that inspired the science, watch the webinar. He joins an expert from Cabaletta Bio to share his experiences using MPA data for lead selection and IND submission. From this webinar, you will:
- Understand how Integral Molecular’s MPA is used to identify potential off-target binding liabilities
- Learn how MPA data supports IND submissions with advantages over traditional tissue cross-reactivity studies
- Discover how the MPA was used to rapidly evaluate the specificity of novel CAR-T cell therapies for autoimmunity and cancer
.png?width=341&height=111&name=perelman(58).png)
.png?width=116&height=60&name=Untitled%20design%20(591).png)
