The Need: High-throughput Zika & dengue neutralization assays
 Takeda is a global biopharmaceutical company developing vaccines against dengue and Zika viruses. Vaccine clinical trials often use the resource-intensive plaque reduction neutralization test (PRNT) that employs live virus to test the immunogenicity and neutralizing serum responses of their vaccine candidates. Takeda describes PRNT assays as a low-throughput, imprecise, manual, labor-intensive assay that represents a major bottleneck in testing large numbers of sera for dengue vaccine and Zika vaccine clinical studies (Bohning et al., 2021). Thus, they sought alternative methods for immunogenicity testing.
Takeda is a global biopharmaceutical company developing vaccines against dengue and Zika viruses. Vaccine clinical trials often use the resource-intensive plaque reduction neutralization test (PRNT) that employs live virus to test the immunogenicity and neutralizing serum responses of their vaccine candidates. Takeda describes PRNT assays as a low-throughput, imprecise, manual, labor-intensive assay that represents a major bottleneck in testing large numbers of sera for dengue vaccine and Zika vaccine clinical studies (Bohning et al., 2021). Thus, they sought alternative methods for immunogenicity testing.
The Solution: Validated Zika and dengue Reporter Virus Particles
To aid in developing Zika and dengue vaccines, Integral Molecular offers Reporter Virus Particles (RVPs) which are replication-incompetent particles that enable assessment of antibody neutralization and antibody-dependent enhancement using high-throughput, plate-based luciferase or GFP assays. RVPs are stable quality-controlled reagents that provide reproducible neutralization titers that are well-correlated with PRNT assays.
Validation data for RVPs representing Zika and all 4 serotypes of dengue have been published by Whitbeck et al., and Mattia et al. All RVPs produced at Integral Molecular are replication incompetent and can be safely used with standard instrumentation found in a BSL-2 laboratory.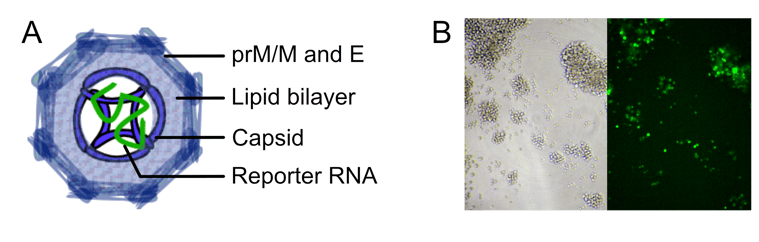
Dengue RVP composition and reporter expression. (A) Schematic representation of RVPs containing the viral structural components pre-membrane/membrane proteins (prM/M), envelope (E), capsid, a lipid bilayer, and reporter RNA. The Reporter RNA encodes GFP or Luciferase, and DENV non-structural proteins responsible for replication of the RNA. (B) Target cells (Raji DC-SIGNR) infected by RVPs expressing GFP are easily visualized by microscopy or quantified by flow cytometry.
The Impact: Advancing vaccine development
Zika vaccine development
As an alternative to live-virus assays, scientists at Takeda developed a high-throughput RVP 384-well microneutralization assay to quantitatively assess neutralizing antibodies in patient serum samples. They validated their RVP neutralization assay as having high levels of precision and reproducibility, and good correlation with live virus assays (Bohning et al., 2021).
Takeda scientists used this assay to study the immune responses produced in response to their purified inactivated Zika virus vaccine candidate TAK-426 in a phase 1 clinical trial (NCT03343626) (Han et al., 2021). In this setting with many patient samples, the RVP assay provided similar results with PRNT with a strong correlation of 0.94 (Bohning et al., 2021). Immunogenicity studies using RVPs showed good initial titers (Han et al., 2021) and persistent seropositivity at timepoints two years post-immunization (Acosta et al., 2022). The vaccine TAK-426 also had a good safety profile, and a phase 2 clinical trial is planned.
.jpg?width=341&height=325&name=(PRNT).jpg)
Takeda assayed patient sera during a Phase 1 clinical trial and showed neutralization titers obtained from PRNT and RVP assays had high correlation.
Dengue vaccine development and approval
Takeda also employed RVPs to characterize serum responses in their phase 2 and phase 3 clinical trials of dengue vaccine TAK-003.
TAK-003 is a tetravalent vaccine consisting of live attenuated dengue-2 virus that also contains structural protein components of dengue-1, -3, and -4 (DeMaso et al., 2022). In dengue vaccine clinical trials, patient sera displayed high serum titers of antibodies against dengue-2. Takeda needed to show that antibodies capable of neutralizing other dengue serotypes were also produced. They accomplished this by depleting patient sera samples of dengue-2 and cross-reactive antibodies, and testing the remaining sera for neutralization of other serotypes using dengue-1, -3, and -4 RVPs. The scientists at Takeda were able to show successful neutralization of all dengue RVP serotypes and thus, that their vaccine induced a broad tetravalent immune response.
Takeda’s dengue vaccine was recently approved in the European Union and Indonesia and is marketed under the name QDENGA.
About Dengue and Zika RVPs
Since 2012, companies have relied on Integral Molecular to provide ready-to-use RVPs for research and development.
RVPs are:- Safe in a BSL-2 environment
- Quantitative (luciferase) or fluorescent (GFP) read-out
- Quality-controlled critical reagents based on 20+ years of virology expertise and experience manufacturing reporter viruses
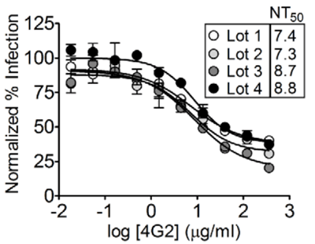
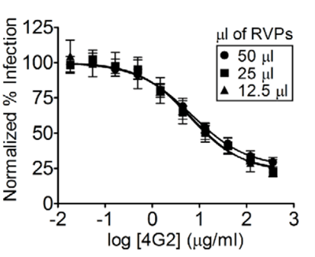
Reproducible Neutralization Titers (Mattia et al.)
- RVPs provide reproducible neutralization titers even across different production lots:
- RVPs provide consistent neutralization titers with different volume inputs:
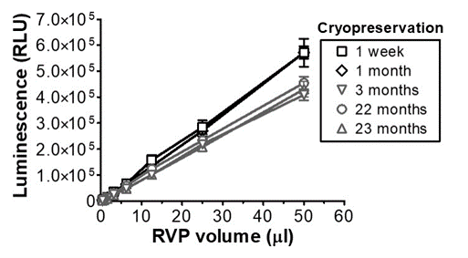
Stability of RVPs (Whitbeck et al.)
- RVPs are stable for years when cryopreserved at -80°C: