The Need
In response to the COVID-19 pandemic, researchers at the University of
Pittsburgh raised a panel of high-affinity SARS-CoV-2 nanobodies. To develop a
potent SARS-CoV-2 neutralizing cocktail for rapid development, the team
required a safe and fast method to screen these molecules for virus-neutralizing
activity.
The Solution
SARS-CoV-2 Reporter Virus Particles Enable Rapid Selection of Potent Neutralizing Nanobodies
Integral Molecular’s quality-controlled SARS-CoV-2 Reporter Virus Particles (RVPs) enabled safe (BSL-2), easy, and high-throughput assessment of SARS-CoV-2 nanobodies. RVP-based neutralization assays enabled the selection of three nanobody candidates with exceptional neutralizing potency.
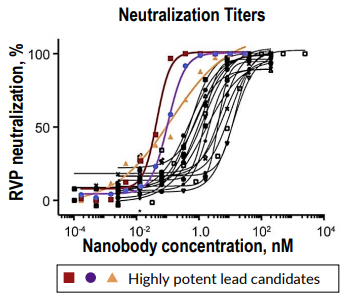
The Impact
SARS-CoV-2 RVP Results Validated Using Live Virus
Fourteen of the most potent antibodies were assayed for neutralization using both luciferase RVPs and live infectious SARS-CoV-2 virus by plaque reduction neutralization testing (PRNT).
RVP neutralization assay results directly correlated with Plaque Reduction Neutralization Tests (PRNT) using live virus, with R2 = 0.92.
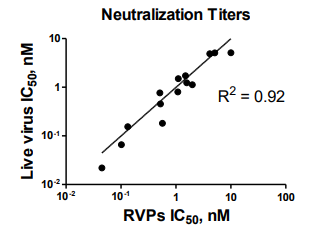
Data featured in Xiang et al., 2020.

